Fa'atauga vevela maualuga CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA)
Fa'atauga vevela maualuga CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA)
Matou te talitonu i taimi uma e filifilia e le tagata le uiga maualuga o oloa, o faʻamatalaga e filifili ai oloa' maualuga, faʻatasi ai ma le REALISTIC, EFFICIENT AND INOVATIVE agaga o le auvaa mo Hot-selling High Quality CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA), O le a matou faia ni taumafaiga faʻateleina e fesoasoani i tagata faʻatau i totonu o le atunuʻu ma faʻavaomalo, ma faʻatupuina le manuia tutusa ma le manumalo-manumalo faigapaʻaga i le va oi matou.o loo matou faatalitali ma le naunautai mo lo outou galulue faatasi faamaoni.
Matou te talitonu i taimi uma e filifilia e le tagata le uiga maualuga o oloa, o faʻamatalaga e filifili ai oloa' tulaga maualuga, faʻatasi ma le REALISTIC, EFFICIENT AND INNOVATIVE agaga auvaa moSaina Mipa ma Monoisopropanolamine, Matou te tuʻufaʻatasia mamanu, gaosiga ma faʻatau atu faʻatasi ma le sili atu i le 100 tagata faigaluega tomai, faʻamalosia lelei faiga faʻatonutonu ma tomai faʻatekonolosi. Matou te tausia sootaga pisinisi umi ma le faʻatau oloa ma tufatufa atu e sili atu i le 50 atunuu, e pei o Amerika, UK, Kanata, Europa ma Aferika ma isi.
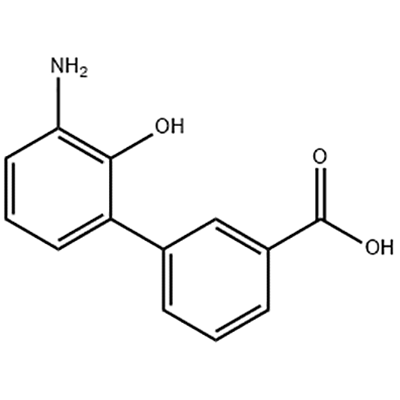
3'-Amino-2'-hydroxy-[1,1'-bipheny]-3-carboxylic acid o loʻo faʻaaogaina e avea ma mea vavalalata o Eltrombopag .
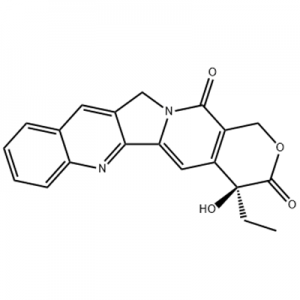
Eltrombopag, atiina ae e GlaxoSmithKline (GSK) i Peretania ma mulimuli ane atiina ae soofaatasi ma Novartis i Suitiselani, o le muamua ma e naʻo le faʻatagaina laiti mole non peptide TPO receptor agonist i le lalolagi.Eltrombopag na faʻamaonia e le US FDA i le 2008 mo le togafitiga o le idiopathic thrombocytopenic purpura (ITP), ma i le 2014 mo togafitiga o le maʻi aplastic anemia (AA).O le vailaʻau muamua foʻi na faʻamaonia e le US FDA mo togafitiga o le AA i le 30 tausaga talu ai nei.
I le december2012, ua faamaonia e le US FDA Eltrombopag mo le togafitiga o thrombocytopenia i tagata mamaʻi ma hepatitis C (CHC), ina ia hepatitis C maʻi ma le le lelei prognosis ona o le maualalo platelet faitau e mafai ona amata ma tausia interferon faavae togafitiga tulaga mo maʻi ate.I le Fepuari3, 2014, GlaxoSmithKline na faʻasalalau e le FDA na tuʻuina atu le agavaa o vailaʻau faʻapitoa mo togafitiga o Eltrombopag mo togafitiga o le hemopenia i tagata mamaʻi e maua i le chemicalbook aplastic anemia (SAA) e leʻi tali atoatoa i le immunotherapy.I le aso 24 o Aokuso, 2015, na faʻamaonia ai e le US FDA Eltrombopag mo togafitiga o le thrombocytopenia i tagata matutua ma tamaiti e taʻi 1 tausaga ma sili atu ma le thrombocytopenia puipuia tumau (ITP) e le lava le tali atu i corticosteroids, immunoglobulins poʻo splenectomy.I le Ianuari 4, 2018, na faʻamaonia ai Eltrombopag e lisiina i Saina mo togafitiga o le thrombocytopenia puipuia muamua (ITP).
Matou te talitonu i taimi uma e filifilia e le tagata le uiga maualuga o oloa, o faʻamatalaga e filifili ai oloa' maualuga, faʻatasi ai ma le REALISTIC, EFFICIENT AND INOVATIVE agaga o le auvaa mo Hot-selling High Quality CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA), O le a matou faia ni taumafaiga faʻateleina e fesoasoani i tagata faʻatau i totonu o le atunuʻu ma faʻavaomalo, ma faʻatupuina le manuia tutusa ma le manumalo-manumalo faigapaʻaga i le va oi matou.o loo matou faatalitali ma le naunautai mo lo outou galulue faatasi faamaoni.
Fa'atauga vevelaSaina Mipa ma Monoisopropanolamine, Matou te tuʻufaʻatasia mamanu, gaosiga ma faʻatau atu faʻatasi ma le sili atu i le 100 tagata faigaluega tomai, faʻamalosia lelei faiga faʻatonutonu ma tomai faʻatekonolosi. Matou te tausia sootaga pisinisi umi ma le faʻatau oloa ma tufatufa atu e sili atu i le 50 atunuu, e pei o Amerika, UK, Kanata, Europa ma Aferika ma isi.